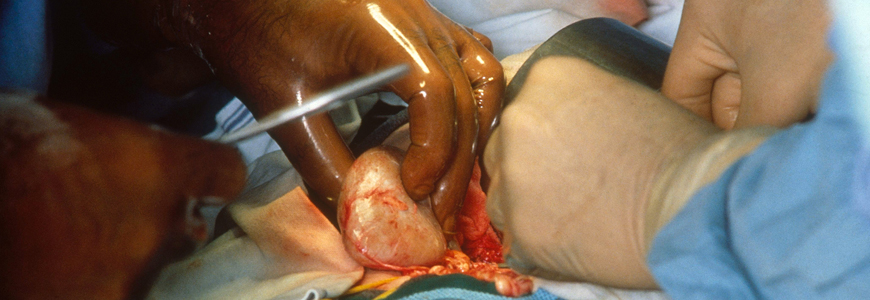
Duke specialists have transplanted more than 60 hepatitis C virus (HCV)-positive kidneys into recipients who did not have the virus as part of a multidisciplinary initiative to address the acute organ shortage.
The HCV-positive (HCV+) organ transplant program has cut the wait time for some recipients in half—from an average of 5 years to 2 to 2.5 years, says Matthew J. Ellis, MD, nephrologist and medical director of the Duke kidney transplant program. Some patients receive organs within a month of being placed on the transplant list, he adds. Blood types affect transplant compatibility and waiting time.
As part of the initiative, patients are counseled about the risk of HCV+ organs and advised about antiviral therapy; they must consent to accepting an HCV+ organ before participating in the program.
The transplants and subsequent elimination of the virus were successful, Ellis says. No patient or allograft losses were reported, he adds, and the glomerular filtration rate (GFR) of the patients who have received transplants averages about 55 mL/min.
“It’s harder to quantify how well the transplanted kidney is doing, but the GFR is about average for patients who do not have HCV,” Ellis says. The antiviral MAVYRET (AbbVie: Chicago, IL), a glecaprevir and pibrentasvir combination taken orally once a day, is used to clear the HCV infection.
The HCV+ organ transplant program is part of a Duke collaboration among the transplant, infectious disease, and hepatology programs. The Duke Institutional Review Board (IRB) created a protocol to oversee monitoring and therapy.
The initiative has resulted in twice as many kidney transplants as that of other organs, says Cameron R. Wolfe, MBBS, infectious disease specialist and IRB principal investigator. Through mid-June, the initiative has resulted in HCV+ organ transplants involving 63 kidneys, 25 hearts, 17 lungs, and 27 livers.
The origin of HCV+ organ transplants dates back more than five years when the FDA approved the first effective antivirals. Shortly after the drugs were approved, two clinical studies assessing HCV+ prompted major transplant centers to examine the feasibility. Those two key clinical trials—the Exploring Renal Transplants using Hepatitis C Infected Donors for HCV-negative Recipients (EXPANDER-1) and Transplanting Hepatitis C Kidneys into Negative Kidney Recipients (THINKER-1)—were influential in advancing pilot programs involving HCV+ transplants.
“For years, when we looked at the data associated with HCV+ kidneys, we understood that the organ did not last as long as an organ that was not infected,” Ellis says. “But the positive effect of cutting the wait time in half is greater than the risk of kidney longevity.” The health of the transplanted organ may prove to be longer than expected, Ellis says, after the virus is cleared.