Study Demonstrates Use of HBOT for Reducing Risk of Central Airway Stenosis
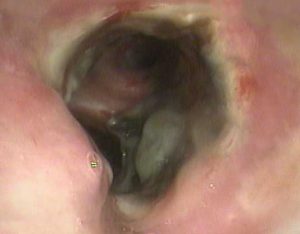
Hyperbaric oxygen therapy (HBOT) can be safely administered to patients at risk for developing central airway stenosis after lung transplant, a group of Duke physicians report in the September 2016 issue of Clinical Transplantation.
Central airway stenosis is common after lung transplant and is defined as the inability to introduce a commonly used 6.3-mm outer-diameter flexible bronchoscope through central airways that can normally be traversed. Central airway stenosis is a cause of significant morbidity and is often preceded by ischemia-related airway necrosis. HBOT, a useful treatment for ischemic skin grafts and poorly healing diabetic wounds, may reduce the development of airway stenosis.
Although it was not designed to assess efficacy, the pilot study is the first to demonstrate the safety of HBOT in patients who develop necrotic airway plaques after lung transplant. HBOT did not decrease the incidence of central airway stenosis in the treatment group compared with the control group, but the need for airway stent placement was lower in study participants treated with HBOT.
“We were working on a hypothesis that airway ischemia plays a major role in patients who develop extensive necrotic airway plaques after lung transplant, as we showed in another study,” says Kamran Mahmood, MD, first author of the paper. “These patients may benefit from therapy that improves oxygen delivery and wound healing.”
The pilot study is the first report on the safety and feasibility of HBOT to manage patients at risk for developing central airway stenosis. It was based on a review of 233 lung transplants performed between March 2013 and January 2016 by the Duke lung transplant program.
Patients with extensive necrotic airway plaques were identified by primary lung transplantation specialists during routine bronchoscopy at 1 to 2 months following transplant. These patients were referred to the interventional pulmonology group to determine whether they were candidates for HBOT. Outcomes of those assigned to HBOT were compared with a contemporaneous reference group with similar plaques who received standard care that did not include HBOT.
The authors reported that the incidence of central airway stenosis was similar between study patients treated with HBOT and reference patients (70% vs 87%, respectively; P = .34), but fewer stents were required in study patients receiving HBOT (10% vs 56%; P = .03).
HBOT was also well tolerated. Ten study patients received HBOT for 18.5 sessions (interquartile range: 11-20), starting at 40.5 days (interquartile range: 34-54) after transplant.
The results of this pilot study are being validated by an ongoing, prospective, randomized controlled trial at Duke University Medical Center (NCT02363959).