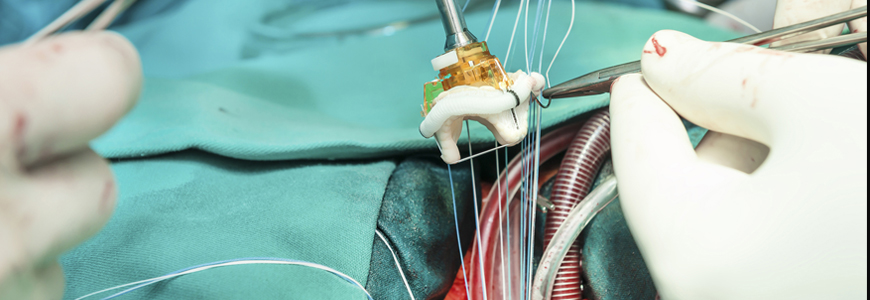
Outcomes from two transcatheter aortic valve replacement (TAVR) trials demonstrated that the nonsurgical option is superior to or at least as effective as surgical aortic valve replacement (SAVR) for patients with aortic stenosis who have a low surgical risk. The simultaneous studies were published in March 2019 in the New England Journal of Medicine (NEJM).
A Duke TAVR specialist predicts the results will lead to FDA approval as well as reimbursement by the Centers for Medicare & Medicaid Services (CMS) for expanded low-risk TAVR applications within a few months. That’s good news for patients who want to avoid the longer, more arduous recovery from SAVR, says J. Kevin Harrison, MD, medical director of Duke’s Structural Heart Disease Program.
But Harrison, who has been a leader of Duke’s TAVR program—one of the first in the nation—sounded a cautionary tone following the NEJM reports. Expanded use of TAVR must be monitored carefully, he says, particularly its use in medical centers that do not conduct the multidisciplinary, team-based anatomical assessments Harrison deems essential for patient safety.
Harrison also encouraged rigorous outcome reviews of the procedure in medical centers that do not have extensive TAVR experience, a concern that was also raised by an unrelated recent analysis from the Duke Clinical Research Institute (DCRI).
Centers Performing Higher TAVR Volumes Have Lower Mortality Rates
The DCRI data, published in April 2019 in NEJM, reported that hospitals performing the highest volume of TAVR procedures have significantly lower mortality rates than centers that perform fewer of the minimally invasive procedures. Duke cardiologist Sreekanth Vemulapalli, MD, lead author of the DCRI study, says the analysis shows a clear relationship between annual TAVR volume and 30-day mortality.
“If the typical approval process unfolds, we will be offering TAVR to this new class of patients within three or four months,” Harrison says. “For patients who want a less invasive option that will put them out of commission for the shortest amount of time, this is truly good news,” Harrison says. “But it is important to monitor outcomes and make sure that every center assesses every patient’s individual anatomic issues.”
The results from the latest trial involving patients at lower surgical risk are significant because TAVR outcomes have now proven superior or equal to SAVR across a broad risk spectrum, Harrison and other specialists say.
“I resist the use of the term ‘low-risk’ because I don’t think there is a true category of low-risk surgery,” Harrison says. “But this data appears to be heralding the phase in which we will be able to offer TAVR to any patient with aortic stenosis, regardless of their open surgical risk, as long as the Duke multidisciplinary team’s detailed anatomic review determines this to be the best treatment strategy for that individual.”
Results from both the PARTNER 3 and EVOLUT studies were summarized by the simultaneous NEJM analyses. The results from Duke were included in the EVOLUT trial publication.