New Advancements in Lupus Treatment
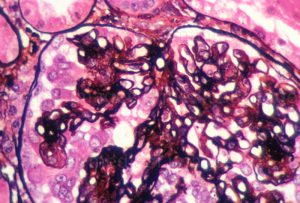
The development of 2 lupus therapies—an experimental immunosuppressant designed to treat lupus nephritis and a monoclonal antibody developed for systemic lupus erythematosus (SLE)—is being closely monitored by rheumatologists anxious to identify new treatments for these challenging disease states.
Voclosporin is an analog of ciclosporin that offers improved metabolic stability in the treatment of lupus nephritis, and anifrolumab is a monoclonal antibody that has proven efficacy for SLE in clinical trials.
“It’s a very significant development in our field to have 2 promising therapies that offer new treatment options for disease conditions as complicated as lupus,” says David Pisetsky, MD, a Duke rheumatologist and researcher. “These therapies have emerged from phase 2 trials successfully and are getting a lot of attention because many products don’t advance in this pipeline, particularly for non–renal-associated lupus.”
Duke rheumatologists closely follow drug development for lupus nephritis, Pisetsky says, because of a regional focus on patients with the disease, which occurs more commonly among African Americans.
“These patients have multiple risks for poorer outcomes during kidney disease treatments,” he explains. “The availability of a new option would be very significant for these patients.”
Both therapies began phase 3 trials during 2017, Pisetsky says, which may result in their availability within 12 months, depending on the trial outcomes.
A total of 324 patients have enrolled in the randomized, controlled, double-blind, 52-week, phase 3 clinical trial of voclosporin. Researchers will assess the efficacy of the drug compared with placebo in achieving complete remission in study volunteers with active lupus nephritis.
A phase 2 trial for anifrolumab concluded in 2016, the results of which were published in the February 2017 issue of Arthritis & Rheumatology. Across multiple clinical end points, the study data suggested that participants with moderate to severe SLE treated with anifrolumab had substantially reduced disease activity compared with those assigned to placebo.