Imaging Innovation: 3-D Surgical Planning for Intractable Epilepsy
Team’s multidimensional approach advances clinical precision, image resolution
To enhance surgical planning for patients with intractable epilepsy, a team of Duke neurologists has created 3-D representations of patients’ own brains—an innovative technique that enables physicians to better visualize the intracranial depth electrodes that monitor electrical activity during and between seizures.
Common 2-D imaging modalities make visualizing relative relationships between electrodes challenging when attempting to isolate a seizure focus. But the 3-D imaging effort, led by Muhammad Zafar, MD, epileptologist and pediatric neurologist, and Abhi Kapuria, MD, neurology resident, uses patient-specific data from MRIs and CT scans to design customized physical and virtual models that accurately represent the position of electrodes within a patient’s cerebrum and their relative relation to one another.
 “This methodology provides a much higher resolution than 2-D imaging and better represents the lesion activity so that we can make a better decision for surgical precision,” Zafar says, noting that Duke plans to include it as a regular part of patient care for presurgical evaluation.
“This methodology provides a much higher resolution than 2-D imaging and better represents the lesion activity so that we can make a better decision for surgical precision,” Zafar says, noting that Duke plans to include it as a regular part of patient care for presurgical evaluation.
Once the neurologists identify where the seizure focus might be based on scalp monitoring, MRIs, and a CT scan, they then implant intracranial depth electrodes in an area of resectable brain that would cause the patient the least amount of harm or result in the least number of neurological deficits and the best reduction of seizure outcome, Kapuria says.
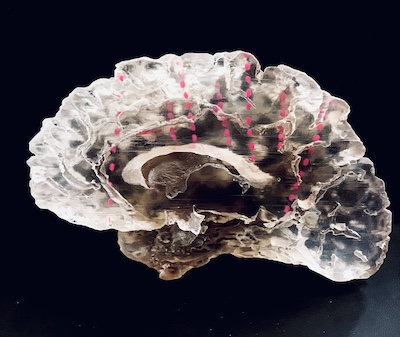
In collaboration with the Innovation Co-Lab and Multi-Dimensional Image Processing Lab at Duke, the team creates a 3-D model by overlaying MRI imaging—which provides the cerebral structure—and the CT scan—which best reflects the metal in the implanted electrodes. The team then uses several layers of programs to reconstruct those data into a meaningful physical model that illustrates the electrodes suspended in a 3-D space.
The final product of this work is a 1:1 physical model of the resection, made of smooth, thick resin polymer. “You can actually hold the patient’s brain, look through it, and approximate where those electrodes intersect,” Kapuria says.
The 3-D models have also been transformed into immersive virtual reality and augmented reality experiences, enabling epileptologists and neurosurgeons to scroll through the brain and zoom into the electrodes they want to examine. “Within a two-dimensional constraint, it is a challenge to demonstrate how these electrodes relate to one another,” Kapuria says.
Zafar and Kapuria’s future work will include additional applications of these techniques for surgical planning in the interactive augmented reality space. They also plan to use the protocols developed in these collaborations to be able to print these models on their own, Zafar says.
