A 60-year-old male presented to functional neurosurgeon Stephen C. Harward II, MD, PhD, with severe essential tremor (ET), a history of hypertension, and von Willebrand disease (VWD) Type 2B.
The patient reported a more than 20-year history of tremor impacting both hands, with his left, dominant hand most affected. Despite medication trials, the patient’s tremors progressed. Upon neurologic exam, the patient scored a 34 on the Clinical Rating Scale for Tremor (CRST). He reported disability with writing, eating with utensils, and drinking out of a cup.
Harward proposed placing bilateral deep brain stimulating (DBS) electrodes into the ventral intermediate nucleus (VIM)—the current standard of care. However, the patient declined DBS. His father, who also had ET and VWD Type 2B, previously underwent DBS surgery and encountered significant hemorrhagic complications, which terminated the procedure.
What innovative solution did Harward offer to treat the patient’s severe ET?
As an alternative to DBS, Harward recommended right-sided magnetic resonance–guided focused ultrasound (MRgFUS) thalamotomy to address the patient’s left-hand tremor, to which the patient agreed.
MRgFUS thalamotomy is a relatively new surgical therapy for ET (approved by the U.S. Food and Drug Administration in 2012) that uses focused ultrasound to create a 4- to 8-mm) lesion in the thalamus without requiring a skin incision or burr hole. While MRgFUS thalamotomy has an excellent safety profile with no reports of intracranial hemorrhage, there are no published cases of procedural outcomes in patients with VWD.
“Because of the patient’s VWD, we collaborated with the Duke Hematology Team to safely deliver MRgFUS,” says Harward. The hematology team obtained baseline von Willebrand Factor (VWF) and factor VIII activity levels, and recommended monitoring VWF activity and utilizing recombinant VWF therapy (VONVENDI, Takeda Pharmaceuticals U.S.A., Inc., Lexington, MA) before and after the procedure to minimize bleeding risks.
“With a hematology plan in place, we used advanced fiber tracking and image-based and atlas-based consensus coordinates to identify two targets for MRgFUS thalamotomy,” says Harward. “Then, the procedure was performed according to plan and resulted in no complications.”
After the procedure, the patient was closely monitored for VWF activity and intracranial hemorrhage. The patient’s VWF factor activity levels remained between 80% and 100%. He was discharged three days after the procedure and continued on the recommended recombinant VWF therapy for 10 days.
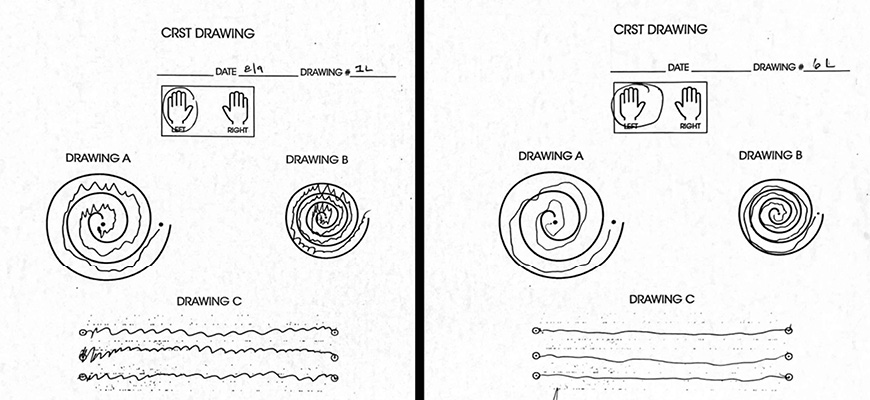
The patient returned for a follow up 12 days after the procedure. He reported 90% improvement of his left-hand tremor, 100% improvement in his quality of life, no side effects, and insignificant bleeding or bruising around his pin sites.
“MRgFUS is a developing technology, and our program is one of the few in the country exploring how to use this technology to treat not only patients with tremor but a variety of other conditions including brain tumors, epilepsy, depression, anxiety, PTSD, and many more,” says Harward.
This image shows the patient's before (left) and after (right) Clinical Rating Scale for Tremor (CRST), demonstrating the marked improvement in handwriting control.
To refer a patient to Duke Neurosurgery, log into Duke MedLink or call 919-943-6411.
