Achieving Complete Response in a Patient With Grade 4 GBM
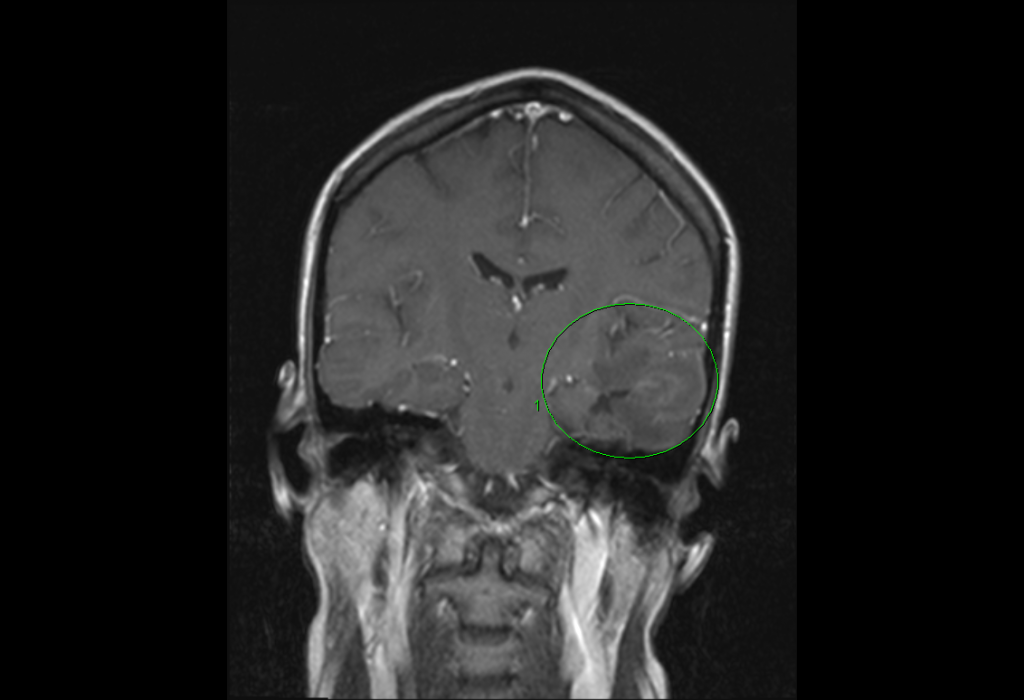
FIGURE 1. Image prior to November 2013 surgery (green circle = tumor area)
A 32-year-old woman presented at Duke Medicine in December 2013 with progressive headache for which she underwent surgery. Duke surgeon Allan Friedman, MD, achieved complete tumor resection of grade 4 glioblastoma multiforme (GBM; Figure 1). The standard of care for patients with grade 4 GBM is 6 weeks of radiation and 6 to 12 cycles of temozolomide, an oral chemotherapy agent. However, even with this treatment, median survival time is approximately 15 months and the 2-year survival rate is 30%.
Question: What experimental therapy can improve rates of complete response (CR) in this patient population when administered postoperatively?
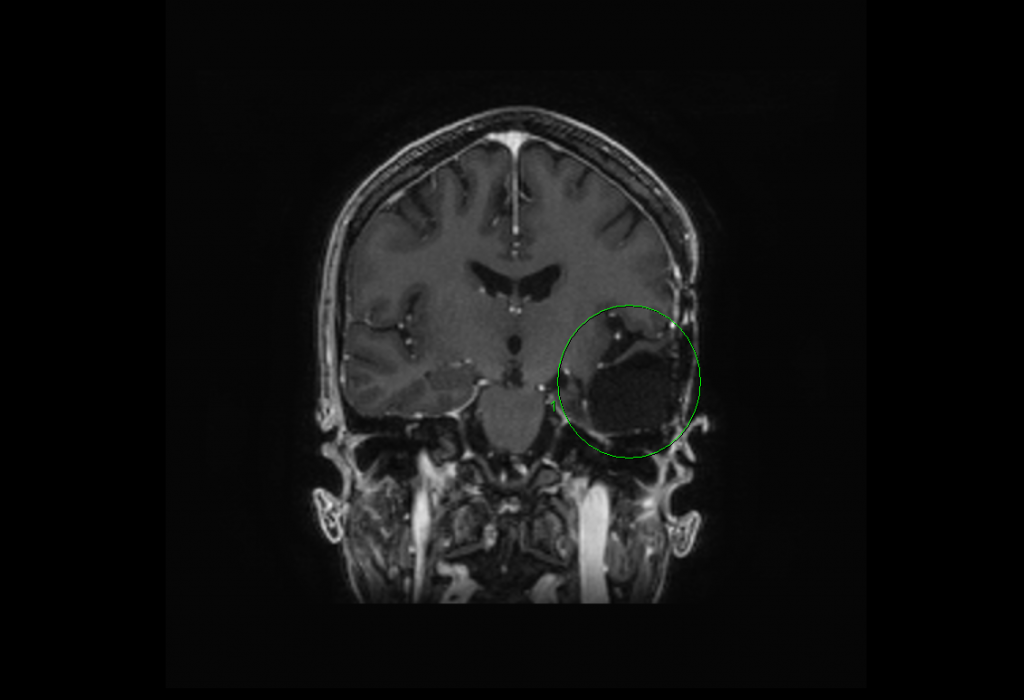
FIGURE 2. Resection cavity from July 2015 (green circle = prior tumor area, now clean)
Answer: The patient received the dendritic-cell (DC) cytomegalovirus (CMV) vaccine series along with basiliximab, a drug that inhibits select regulatory T cells.
Following the surgery, Gordana Vlahovic, MD, enrolled the patient in the REGULATe (REGULATory T-Cell Inhibition With Basiliximab During Recovery From Therapeutic Temozolomide-induced Lymphopenia During Antitumor Immunotherapy Targeted Against Cytomegalovirus in Patients With Newly-Diagnosed Glioblastoma Multiforme) clinical trial in January 2014. As a study participant, the patient received standard radiation and temozolomide as well as a series of 8 CMV vaccines plus basiliximab.
The theory underlying the treatment is that approximately 90% of glioblastomas contain CMV, which is not present in surrounding brain tissue. The vaccine is designed to switch on the immune system by using DCs engineered to be loaded with CMV antigens because these cells “train” the cytotoxic T cells of the immune system to respond to specific antigens—in this case, a protein in CMV—and, by association, target the glioblastoma cells. “The cytotoxic T cell is going to destroy the cancer cell, and it is going to be targeted, because no other tissues should be CMV positive,” explains Vlahovic.
Basiliximab helps in this process because it inhibits specific regulatory T cells that GBM uses to suppress the cytotoxic immune response that target and kill tumor cells.
This patient’s response has been very encouraging. “She is doing well,” Vlahovic comments. “From an oncology standpoint, she has no signs clinically or radiographically of any disease at this point,” she adds (Figure 2). The patient has a new full-time job and is the mother of 2 young children. She has been free of any detectable disease for 21 months.
Various immunotherapy approaches like this one are leading to great strides in the treatment of GBM, and Duke Medicine is a leader in bringing them to patients.
For example, to improve the immunopotency of the DC CMV vaccine, Duke researchers are attempting various approaches to encourage the DCs to travel to the lymph nodes to spur the immune response. Researchers plan on using a tetanus shot to precondition the DC CMV vaccine and improve the migration of the vaccine to the local lymph nodes, where it presents GBM-specific CMV antigens and results in a cytotoxic immune response. Preconditioning with a tetanus shot will be incorporated into a follow-up trial called ELEVATE (Evaluation of Overcoming Limited Migration and Enhancing Cytomegalovirus-specific Dendritic Cell Vaccines With Adjuvant TEtanus Pre-conditioning in Patients With Newly-diagnosed Glioblastoma) for patients with newly diagnosed GBM.
Vlahovic is currently enrolling patients in ELEVATE and other trials, and cutting-edge treatments are available to appropriate patients at the Preston Robert Tisch Brain Tumor Center at Duke.