 Immediately after a baby was born, her care team noticed enlargement and clouding of her corneas. An ophthalmologist and glaucoma specialist in her home state of New York recognized the signs of congenital glaucoma. Having little experience working with pediatric glaucoma, they consulted with Duke pediatric glaucoma specialist Sharon Freedman, MD. Freedman confirmed the diagnosis, and the patient was referred to the Duke Eye Center.
Immediately after a baby was born, her care team noticed enlargement and clouding of her corneas. An ophthalmologist and glaucoma specialist in her home state of New York recognized the signs of congenital glaucoma. Having little experience working with pediatric glaucoma, they consulted with Duke pediatric glaucoma specialist Sharon Freedman, MD. Freedman confirmed the diagnosis, and the patient was referred to the Duke Eye Center.
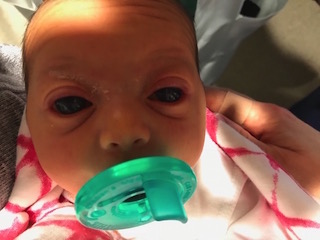
The patient had one of the most severe cases of congenital glaucoma Freedman had ever seen. “It’s not common to see this condition in a newborn,” she says. “The cornea was so big you couldn’t see any sclera, and the corneas were so cloudy you couldn’t see the iris.”
Freedman knew she needed to perform surgery as soon as possible to reduce the risk that the infant’s cloudy corneas would prevent her from learning to see. However, as Freedman warned the family, her first choice surgery to open the trabecular meshwork and Schlemm Canal—trabeculotomy—might not work because of the severity of the glaucoma. In that case, she would implant an artificial drainage device.
As Freedman had anticipated, her attempt at illuminated microcatheter–facilitated 360-degree trabeculotomy in the left eye was unsuccessful; she could find the Schlemm canal but was unable to cannulate fully, she suspects either because the canal was malformed or because the anatomy of the drainage angle was abnormal.
Rather than trying trabeculotomy in the right eye, Freedman implanted a valved glaucoma drainage device (GDD). The challenge with using these devices, she says, is that in congenital glaucoma, the eye stretches nonuniformly, but GDDs are available in only pediatric or adult sizes. This patient’s corneal diameter was extremely large for a newborn at 13 mm, but the axial length of her eyes was ~20 mm, only mildly enlarged comparatively. Whereas a pediatric-sized implant would likely be too small for long-term pressure reduction, an adult-sized GDD would risk contact with the optic nerve.
To address this problem, Freedman and her former trainee developed a formula based on theoretical and confirmatory studies in pediatric autopsy eyes to guide placement of an adult GDD for pediatric or short eyes to avoid contact between the optic nerve and the posterior edge of the GDD (Published here: https://www.jaapos.org/article/S1091-8531(17)30023-X/pdf).
For this patient, in addition to trimming approximately 5 mm off the GDD plate, Freedman placed the implant in the inferior nasal quadrant, leaving the more spacious superior temporal quadrant for later implantation of a larger GDD once the eye had grown if/when the initial GDD was unable to control pressure.
The next day, the patient was doing well, so the family went home. For the next several weeks, she had follow-up visits with her glaucoma specialist in New York. The right eye, which had received the GDD, recovered well. There was marked clearing of the cornea and noticeably less eye bulging.
Not unexpectedly, the left eye still had high pressure, so the patient returned to Duke for a GDD to be placed. During the procedure, Freedman also examined the right eye, determining that the axial length had decreased by 2 mm. Ultrasound confirmed that the fluid-filled capsule surrounding the inferior nasal GDD was still approximately 3 mm from the optic nerve.
 The patient will return to Duke in three months, so Freedman can make sure her eyes are doing well and that the GDDs are well positioned and functioning adequately. Because the patient has multiple Haab striae in both corneas due to her glaucoma, her vision will be permanently affected, Freedman says, stressing the importance of consistent follow-up with the patient’s local ophthalmologist and glaucoma specialist to ensure adequate pressure control. However, her optic nerves appear quite healthy at this point and her corneas are much clearer, so she anticipates the patient’s corrected vision could reach 20/80 or better.
The patient will return to Duke in three months, so Freedman can make sure her eyes are doing well and that the GDDs are well positioned and functioning adequately. Because the patient has multiple Haab striae in both corneas due to her glaucoma, her vision will be permanently affected, Freedman says, stressing the importance of consistent follow-up with the patient’s local ophthalmologist and glaucoma specialist to ensure adequate pressure control. However, her optic nerves appear quite healthy at this point and her corneas are much clearer, so she anticipates the patient’s corrected vision could reach 20/80 or better.
One month after the second surgery, the baby’s vision is beginning to develop. “She has a social smile now,” Freedman reports. “It’s like she’s a different child.”

